Aramid is a strong fiber derived from highly aligned aromatic benzene rings connected with amide (−CO−NH−) bonds. Para-aramid (“Kevlar,” “Twaron,” “Heracron,” “Alkex,” and others,) and meta-aramid (“Nomex,” “Conex,”) differ in the location of their amide bonds. The terms “para” and “meta” refer to the positions of the amide linkages relative to each other on the aromatic rings: in para-aramid, the linkages are directly opposite each other (1,4-substitution), while in meta-aramid, they are adjacent (1,3-substitution).
A para-aramid fiber’s molecular structure looks like the image below. I’ve diverged from usual chemical structure representations, which would have the amide bonds arranged in a zig-zag, as in this case a rigid rod more accurately represents its structure. In a single aramid fiber, millions of the units depicted below – repeat units – are strung along in a long chain.

Meta-aramid – Nomex – is, in contrast:

Para-aramid fibers exhibit a higher degree of molecular orientation and crystallinity compared to meta-aramid fibers. In para-aramid, the linear rigid-rod structure of the polymer chains allows them to pack closely together in a highly ordered crystalline arrangement. This facilitates extensive hydrogen bonding between the amide groups of adjacent chains and strong π-π stacking interactions between the aromatic rings. These interactions result in a highly aligned bulk structure, and the properties of the material are downstream of its structural characteristics.
On the other hand, the Nomex/meta-aramid structure introduces a kink in the polymer chain due to the adjacent substitution pattern. This kink reduces the ability of the chains to align as closely and orderly as in para-aramids, leading to lower crystallinity and, consequently, lower strength. The less ordered structure of meta-aramid fibers still allows for hydrogen bonding, but the overall intermolecular interactions are weaker compared to those in para-aramids.
It’s generally said that the strength of a strong inorganic fiber – such as fiberglass – can approximate the strength of its atomic bonds if it’s substantially free from defects. Para-aramid extends this concept by leveraging additional strength derived from steric interactions between adjacent polymer chains. For this reason, it’s a strong fiber for reasons that go beyond defect minimization.
When para-aramid was first commercialized, in the 1970s, the US Military was making extensive use of ballistic nylon and fiberglass in armor systems. Para-aramid, far stronger than nylon and far lighter than fiberglass, was quickly adopted as a replacement for those materials in many systems and programs, most notably in the PASGT program of the 1980s, which resulted in the issue of helmets and flak vests made of Kevlar-29.
In the years since, numerous different grades of Kevlar have been introduced. In all cases, the structure of the polymer and its fundamental characteristics are unchanged, but modifications to processing techniques have incrementally improved its functional properties. These modifications broadly fall into three categories:
(1) Enhancing molecular orientation. The tensile strength and modulus of Kevlar aramid fibers are directly related to the degree of molecular orientation along the fiber axis. Higher drawing forces during the spinning process align the polymer chains more closely in the direction of the fiber, enhancing the material’s mechanical properties. Different grades of Kevlar are produced by varying the draw ratio during fiber spinning.
(2) Enhancing crystallinity. The extent of crystallinity, or the orderliness of the polymer chains within the material, affects its density, strength, and thermal properties. Controlled heat treatment and stretching of the fibers after spinning can increase crystallinity, leading to stronger and stiffer yarns and fibers.
(3) Finishing. Kevlar fabrics are sometimes treated with a chemical emulsion to reduce friction. In composite applications, they can be chemically treated to increase fiber roughness, which improves adhesion between Kevlar fibers and matrix resins.
To date, chemical modification of the molecular backbone has not been attempted. At least, not in the USA.
Russian Aramid
The so-called “Russian aramids” represent a unique class of materials, which are similar to the aramid fibers and fabrics familiar to us from Kevlar and Twaron, but far from identical. These Russian aramids were sold, until recently, under several trade-names, most commonly “AuTx,” but also “Ruslan” and “Artec.” They are copolymers – copolyamides – consisting of para-substituted phenylene and benzimidazole. Their structure is shown below:
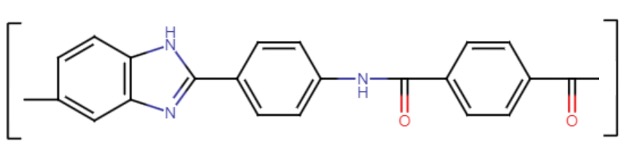
Aramid Derivatives
The benzimidazole component, at the left of the image, is derived from fused benzene and imidazole rings, hence its name. This heterocyclic benzimidazole moiety makes the polymer chain asymmetrical – as you can see, there are two ways for the polymer chain to continue from the benzene side of the benzimidazole ring, but neither of them are perfectly on-center – and this keeps it from locking into a three-dimensional crystal structure, which in turn allows for greater draw ratios during production. Further, the benzimidazole groups contribute to stronger hydrogen bonding and enhanced molecular orientation compared to traditional para-aramid fibers. Stronger hydrogen bonds and improved alignment at the molecular level lead to higher tensile strength and stiffness.
Structurally, it’s obvious that this “aramid” has little in common with what’s typically known as aramid. In fact, it more closely resembles a Zylon/Kevlar hybrid. It’s also reminiscent of the much-hyped but never commercialized M5 Fiber. For both Zylon and M5 also feature a large fused heterocyclic ring bonded to a benzene ring.
Whereas Zylon utilized oxazole moieties, later unfortunately shown to be rather unstable, both M5 and this aramid derivative make use of stable imidazole moieties. The difference – along with the similarities all three materials exhibit – is shown below.
(And, yeah, you can try to make a “stable Zylon” with imidazole rings. Others have apparently already tried. It’s a complicated synthesis that doesn’t seem to turn out well. But if you’re a researcher and are interested in investigating strong fibers, contact the author.)
As one would expect of a fiber that combines the structural characteristics of aramid with those of Zylon/M5, these “Russian aramids” perform extremely well. In a 2011 experiment performed by the US Army Research Labs, AuTx fibers were compared to DuPont Kevlar KM2 fibers. AuTx was found to be more than 40% stronger, 20% stiffer, and nearly 15% more elastic under strain than KM2. Ballistic results were not published, it is known that AuTx ballistic fabrics exhibit a markedly superior performance-to-weight ratio – and it’s said that they can exhibit ballistic weight efficacy on par with UHMWPE.
As of this writing, due to the war in Ukraine and sanctions on Russian military equipment. Producers, AuTx and similar fibers are no longer commercially available. In years past, when they were available, they were extremely expensive. It was claimed that this reflected the scarcity, relative complexity, and very high cost of the chemical required for its synthesis, namely 5(6)-amino-2-(p-aminophenyl)benzimidazole.
Future directions
The chemical modification of polyethylene, short of deuterating it, is effectively impossible. The chemical modification of inorganics such as fiberglass and carbon fiber is naturally tightly constrained. But the chemical modification of aramid and related organic rigid-rod. polymers is very fertile ground for improved armor materials. And one that offers considerable freedom for experimentation.
Future directions in the research and development of modified aramids and post-aramids. Rigid-rod fibers might include the replacement of imidazole with thiazole or less common heterocycles. The below, for instance, is stable and would likely exhibit good mechanical properties:

Or one could take a page from Vectran. And realize that simple fused aromatic rings – naphthalene rings – might also have a place:
And, of course, additional monomers could also be introduced; there’s nothing to prevent repeat units from growing larger.
There’s lots of room to maneuver in aramid chemistry. Recent incredible advances in UHMWPE processing and performance don’t mean aramid is obsolete. It means that the unfathomably deep chemical space of aramid and its analogs must continue to be explored.










